Gold atom hires stock photography and images Alamy
The molar mass of gold is 197 grams per mole. (A) 6.70 times 10 to the 22nd atoms. (B) 7.50 times 10 to the 24th atoms. (C) 4.83 times 10 to the 22nd atoms. (D) 3.63 times 10 to the 23rd atoms. Or (E) 4.41 times 10 to the 23rd atoms. To answer this question, we need to determine the number of gold atoms in a given mass of gold.. See the explanation. One mole of anything, including atoms, is 6.022xx10^23 (Avogadro’s number) of them. Usually you will have a given mass of an element. There are two basic steps to get from the given mass to the number of atoms. They are: “Mass”rarr”Moles” and “Moles”rarr”Atoms” The following example will show you how to do that. ~~~~~ Example: How many atoms of gold are in “58.27 g” of gold?
:max_bytes(150000):strip_icc()/element-list-names-atomic-numbers-606529_FINAL_LARGE-35fc6ad6159646a7937ca0b51220d3b3.png)
Element List Atomic Number, Element Name and Symbol

Calculate the number of atom in 39.4 g gold. Molar mass of gold is `197 g “mole”^(1)` YouTube
Oro (Au) de metal, la estructura cristalina. Los átomos son representados como esferas con

Calculate the number of gold atoms present in 98 5g of gold (atomic mass of gold = 197g

How Many Atoms Are In Gold Gold Periodic Table Protons Bitcoin Sec News

Unit cell of gold crystal is constituted of 4 gold atoms. Download Scientific Diagram

How Many Atoms Are In Gold Gold Periodic Table Protons Bitcoin Sec News

Calculate the number of atoms of Gold in 1g of Gold? (Atomic mass 197g/mol) YouTube

Gold, atomic structure Stock Image C018/3760 Science Photo Library

The number of gold atoms in 300 mg of a gold ring of 20 carat gold ( Au = 197 pure gold is
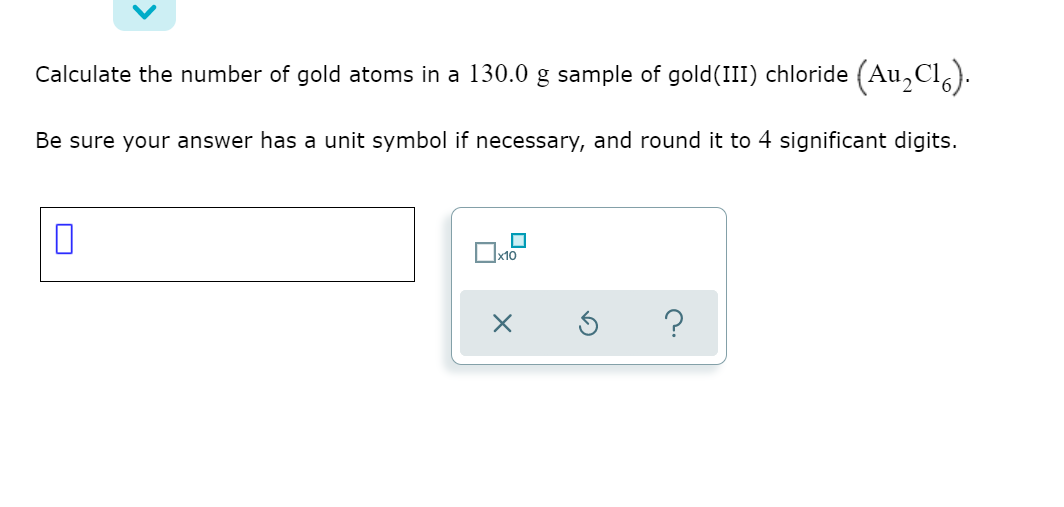
Solved Calculate the number of gold atoms in a 130.0 g
Gold Valence Electrons Gold Valency (Au) with Dot Diagram

Quantum Number Periodic Table Chemogenesis
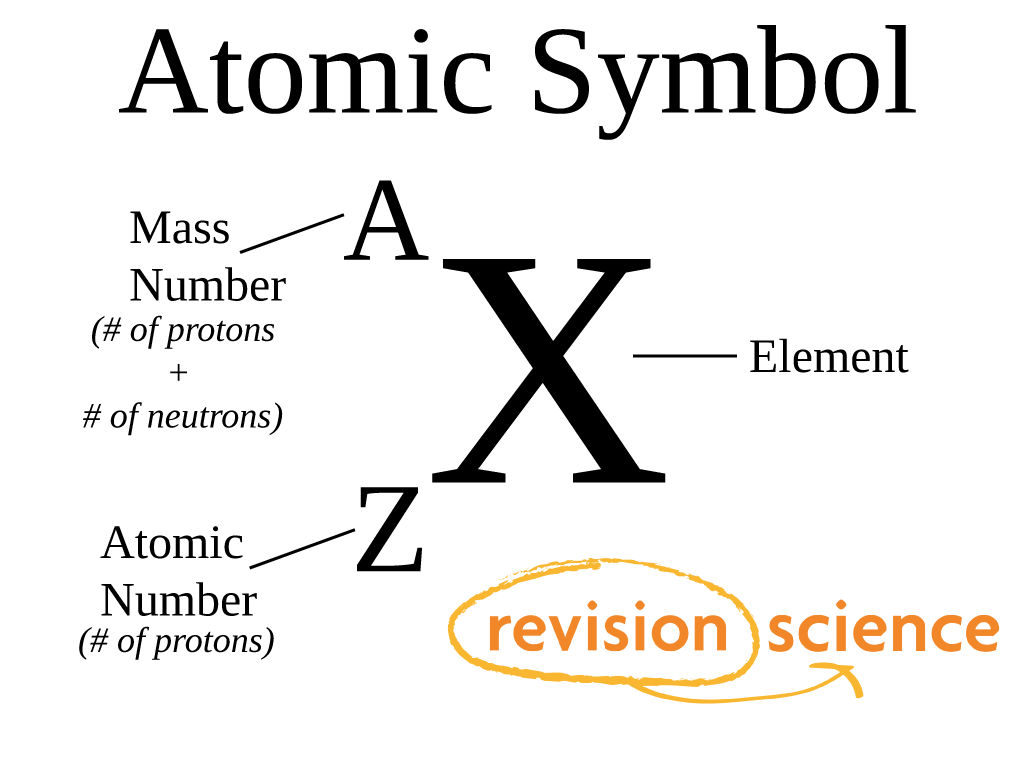
Atomic Number For Gold Nabigh Reswara
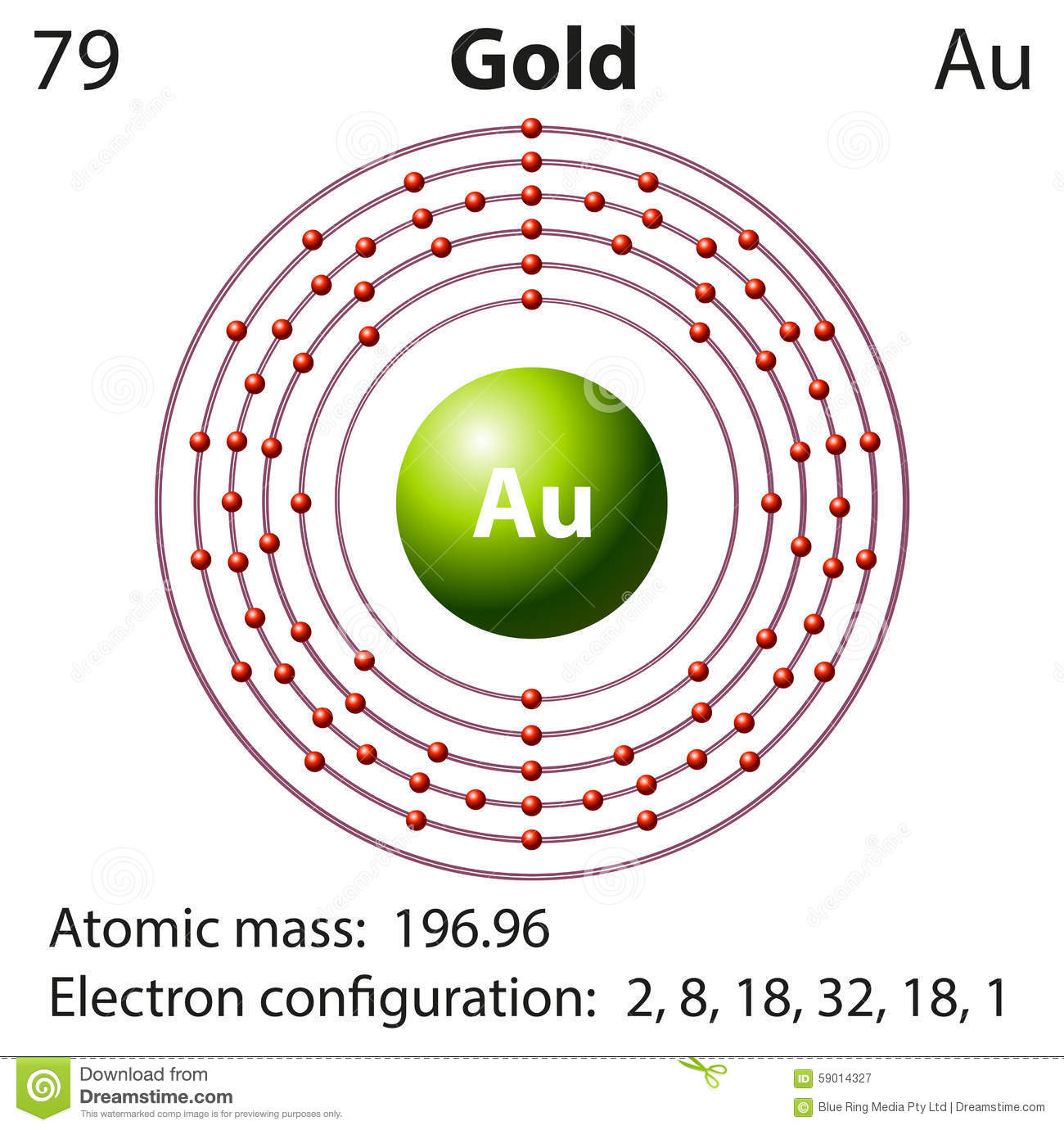
How To Find Elements Electron Configuration For Gold (Au)

Gold, atomic structure Stock Image C013/1636 Science Photo Library
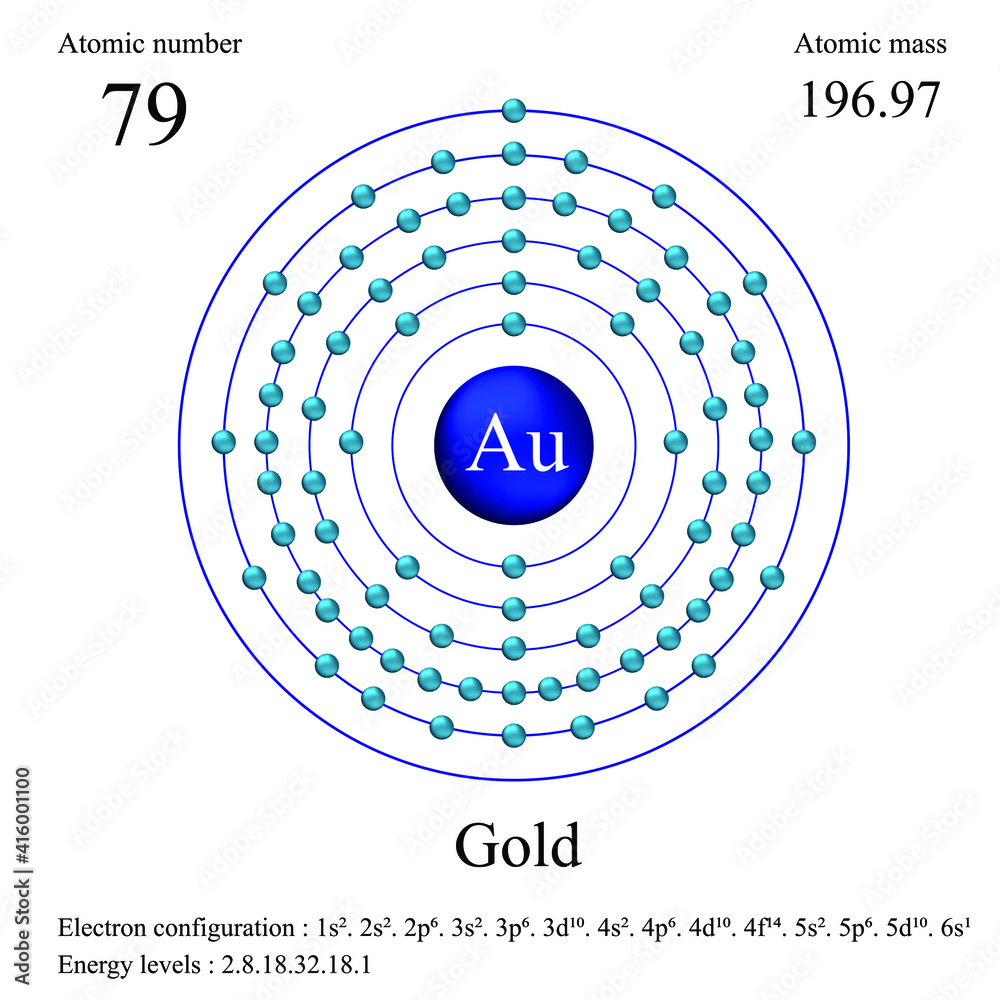
Gold atomic structure has atomic number, atomic mass, electron configuration and energy levels

How Many Atoms Are In Gold Gold Periodic Table Protons Bitcoin Sec News

Calculate the number of atoms in 39.4 g gold. Molar mass of gold is 197g mole^1. Brainly.in

3d render of atom structure of gold isolated over white background Protons are represented as
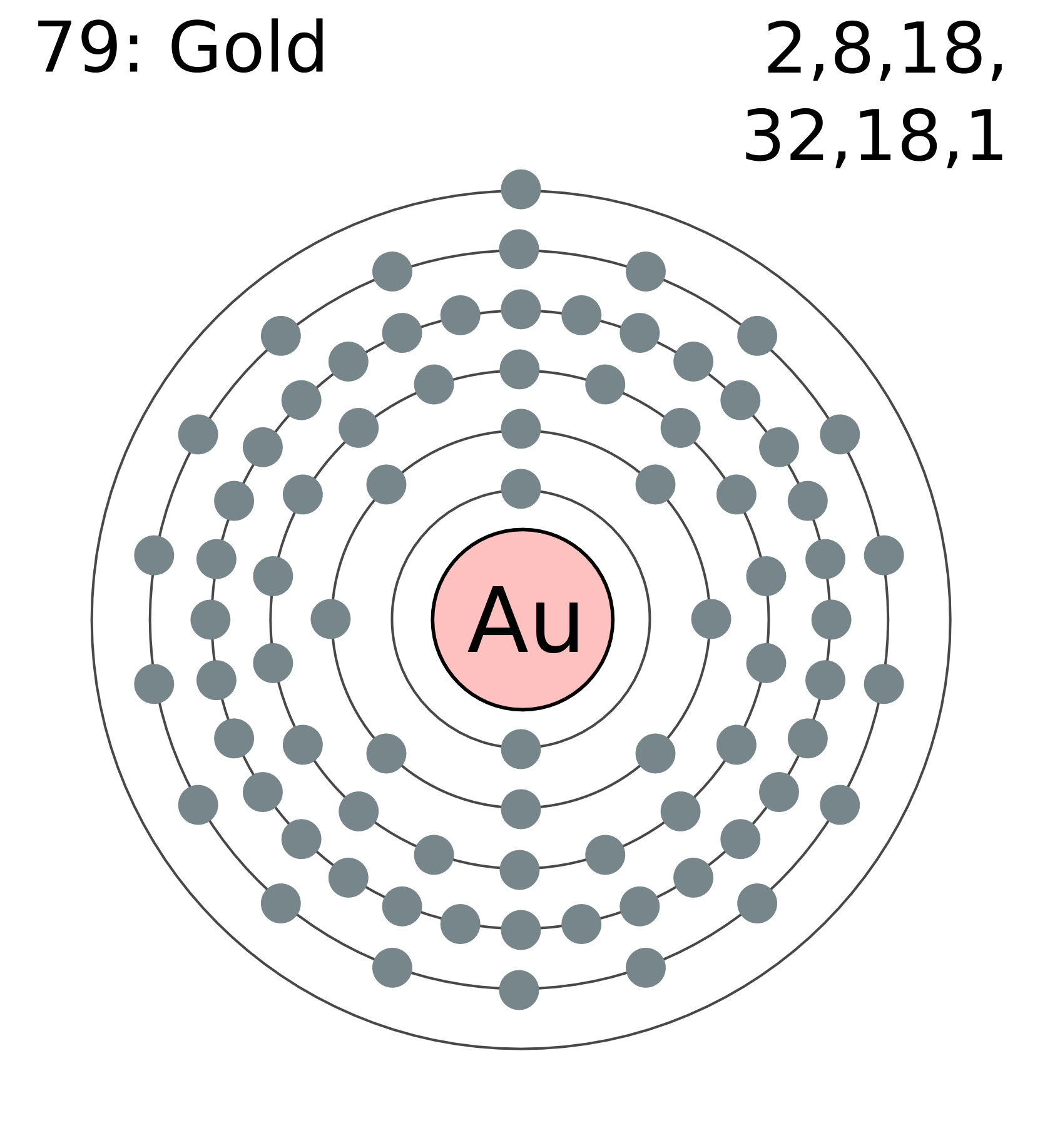
Atomic Number – Protons, Electrons and Neutrons in Gold. Gold is a chemical element with atomic number 79 which means there are 79 protons in its nucleus.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.. Atoms on a corner are shared by eight unit cells and hence contribute only 1 8 1 8 atom per unit cell, giving 8× 1 8 1 8 =1 Au atom per unit cell. The total number of Au atoms in each unit cell is thus 3 + 1 = 4. Exercise 1. Metallic iron has a body-centered cubic unit cell (part (b) in Figure 12.5).